2005년03월06일 37번
[대기열역학] 건조공기의 분자량을 Md, 수증기의 분자량은 Mv 라 할 때 Mv/Md 의 값은?
- ① 0.286
- ② 0.622
- ③ 0.610
- ④ 1.609
(정답률: 알수없음)
문제 해설
Molecular weight of dry air (M_d) is approximately 28.97 g/mol and the molecular weight of water vapor (M_v) is approximately 18.02 g/mol. Therefore, the ratio of M_v to M_d is:
M_v/M_d = 18.02 g/mol / 28.97 g/mol ≈ 0.622
This value is known as the "molar fraction" or "mole fraction" of water vapor in dry air. It represents the fraction of the total number of molecules in a given volume of air that are water vapor molecules.
M_v/M_d = 18.02 g/mol / 28.97 g/mol ≈ 0.622
This value is known as the "molar fraction" or "mole fraction" of water vapor in dry air. It represents the fraction of the total number of molecules in a given volume of air that are water vapor molecules.


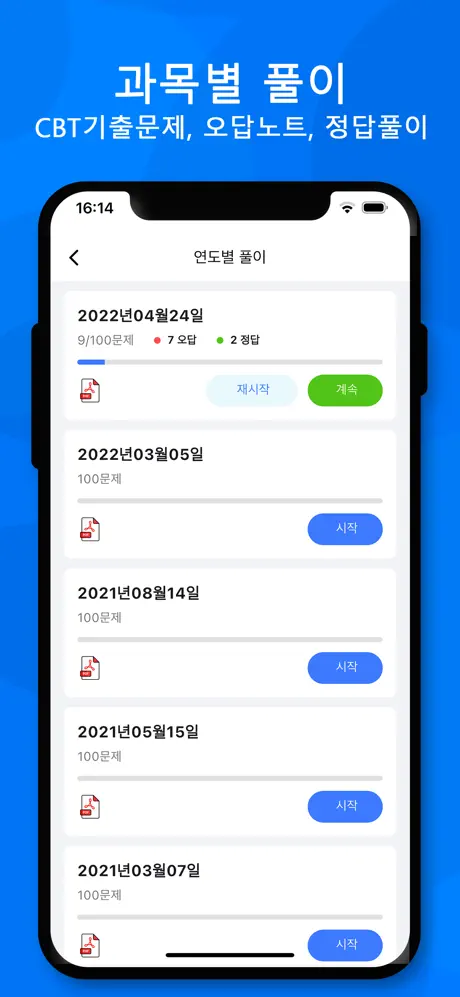
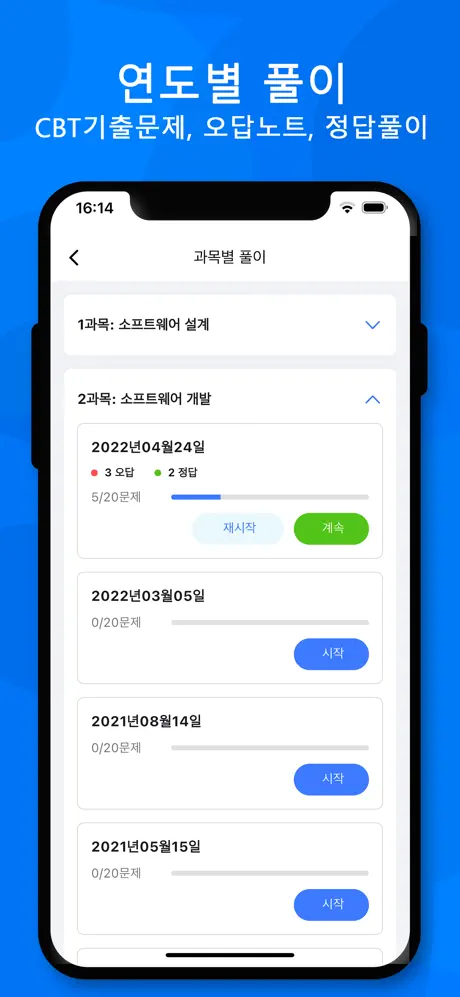
연도별
진행 상황
0 오답
0 정답